Product Overview
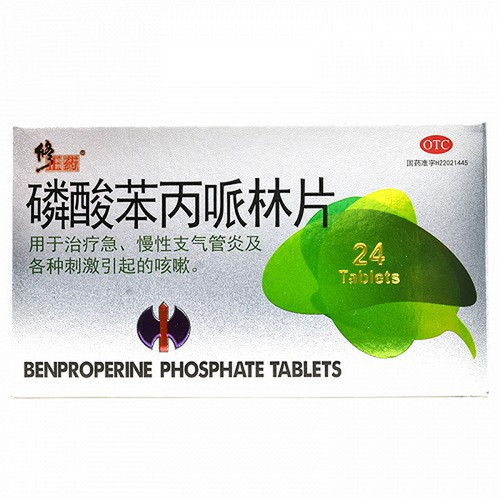
[Drug Name]
Generic Name: Benproperine Phosphate Sustained-Release Tablets
Trade Name: Hebang Benproperine Phosphate Sustained-Release Tablets, 40mg*8 Tablets
Pinyin Code: HeBang LinSuanBenBingZuoLinHuanShiPian 40mg*8Li
[Main Ingredients]
Each tablet contains 52.7mg of the main ingredient, benproperine phosphate. Excipients include starch, hypromellose, cellulose, microcrystalline cellulose, lactose, talc, magnesium stearate, and a special film-coating material.
[Properties]
This product is a film-coated tablet that appears white or off-white after removal of the coating.
[Indications/Main Functions]
For the treatment of acute and chronic bronchitis and coughs caused by various irritants.
[Specifications]
40mg*8 tablets
[Dosage and Administration]
Oral administration: 1 tablet twice daily.
[Adverse Reactions]
Transient oropharyngeal numbness may occur after taking this medication. Other adverse reactions include fatigue, dizziness, upper abdominal discomfort, loss of appetite, and rash.
[Contraindications]
This product is contraindicated if you are allergic to it.
[Precautions]
1. This product has only a cough suppressant effect. If symptoms do not improve significantly after 7 days of use, consult a physician or pharmacist immediately.
2. Use with caution during pregnancy.
3. The tablet must be swallowed whole; do not crush or dissolve it before taking.
4. Consult a physician or pharmacist for dosage for children.
5. This product has no expectorant effect. If expectoration is significant, do not use it.
6. If a rash develops during medication, discontinue use and consult a physician or pharmacist.
7. This product is contraindicated if you are allergic to it. Use with caution if you have allergic reactions.
8. Do not use if the product's properties change.
9. Keep out of reach of children. 10. Children must be supervised by an adult before use.
11. If you are taking other medications, consult your physician or pharmacist before using this product.
[Drug Interactions]
Drug interactions may occur if used concurrently with other medications. Please consult your physician or pharmacist for details.
[Pharmacology and Toxicology]
This product is a non-narcotic antitussive that primarily blocks afferent sensory nerve impulses to pulmonary and pleural receptors, while also directly inhibiting the antitussive center. It also exhibits papaverine-like smooth muscle antispasmodic effects. The sustained-release formulation slowly releases the drug through a specialized mesh, maintaining its effect for up to 12 hours while minimizing gastric irritation.
[Pharmacokinetics]
[Storage]
Store in a sealed container away from light.
[Packaging]
Packaged in aluminum foil, 8 tablets/box.
[Expiry Date]
36 months
[Approval Number]
National Medical Products Approval No. H20041434
[Manufacturer]
Hubei Zhongjia Pharmaceutical Co., Ltd.